We performed the research on clinical and regulatory pharmaceutical sciences.
Regulatory science (RS) is the scientific foundations for the regulatory system through which governments regulate the activities of businesses, particularly those related to the public health or safety. RS is a field of research that is distinct from regulatory affairs, which refers to the actual implementation or enforcement of regulations by government or industry. RS is an interdisciplinary field of natural and social sciences that studies the standards or criteria for regulating medical products, ensuring their safety, efficacy, and reasonableness.
Clinical Pharmaceutical Science (CPS) is a research discipline that conducts the pharmaceutical study at the interface of the bench and bedside. CPS utilizes contemporary research approaches to generate new knowledge applicable to the disposition and activity of drugs in humans and to evaluate differences in drug response among individuals. CPS links clinical pharmacotherapy expertise with bench or mathematical approaches. CPS research projects integrate laboratory techniques, non-clinical models, and human-based research to answer mechanistic questions that improve health outcomes.
Ph.D. in Clinical Pharmaceutical Sciences (2001), University of Pittsburgh
Pharm.D. in Transplant Medicine (1996), University of Minnesota
M.S., in Pharmaceutical Chemistry (1986), Seoul National University
B.S.Pharm., in Manufacturing Pharmacy (1984), Seoul National University
Professor, Regulatory and Clinical Pharmaceutical Sciences (2015. 09 – present), Seoul National University
Professor, Clinical Pharmacy and Regulatory Sciences (2012.03 – 2015.08), Yonsei University
Scientific Investigator (2011 – 2012), U.S. Food and Drug Administration
Clinical Pharmacology Reviewer/Team Leader (2001 – 2011), U.S. Food and Drug Administration
Research Scientist/Clinical Team Leader (1986 – 1992), Lucky Central Research Institute
Committee Member for Regulation Confirmation (2023.03 – present), Ministry of Food and Drug Safety
Vice President (2017 – present) Korean Society of Food, Drug and Cosmetics Regulatory Sciences
Vice President (2015 – present) Korean College of Clinical Pharmacy
Advisory Committee Member for Drug Product Evaluation (2014 – present), National Institute of Food and Drug Safety Evaluation
Member for Central Pharmaceutical Affairs Counsel (2014 – present), Ministry of Food and Drug Safety
Member of the Board of Directors (2014 – 2022), Korean Foundation for Rare Disease
- Research on drug exposure-response relationships
- Discovery and development of protein kinase inhibitors
- Study on improving biopharmaceutic properties of orally administered drugs
- Research on rare diseases and orphan drugs
- Study on drug evaluation and approval
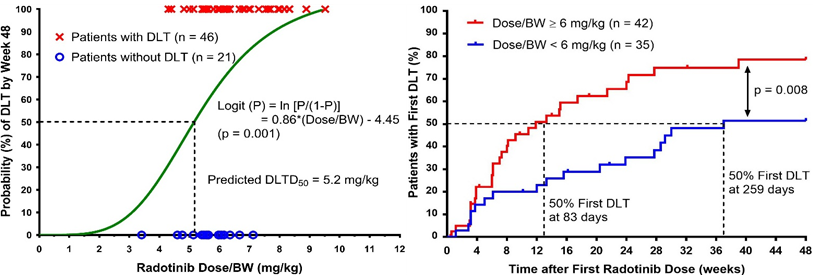
An optimization of radotinibdoses in the treatment of chronic myelogenous leukemia is an example of clinical pharmaceutical research based on both CPS and RS principles. The magnitude of radotinibdose adjusted for patient’s body weight (Dose/BW) and the probability of dose-limiting toxicity (DLT) demonstrated a positive association. There exists a significant difference in the Kaplan-Meier curves for the time to first DLT between the patient subgroups of Dose/BW <6 and ≥6 mg/kg. Hence, a two-tier weight-based dosing regimen has been recommended in a regulatory approval in order to improve the safety profile of radotinib: 400 mg or 500 mg once daily for patients weighing ≤65 or <65 kg, respectively.
- Volumetric absorptive microsampling for the therapeutic drug monitoring of everolimus in patients who have undergone liver transplant. Therapeutic Drug Monitoring. 2023;45(2):223-8.
- Orphan drug designation: a comparison of regulatory systems among United States, European Union, and Republic of Korea. Regulatory Research on Food, Drug & Cosmetic. 2022;2:137-49.
- Appropriate starting dose of dasatinib based on the analyses of dose limiting toxicities and molecular responses in Asian patients with chronic myeloid leukemia. Clinical Lymphoma, Myeloma & Leukemia Research. 2021;21(6):e521-9.
- Development of a limited sampling strategy for the estimation of isoniazid exposure considering N-acetyltransferase 2 genotypes in Korean patients with tuberculosis. Tuberculosis 2021;127:102052.
- Development of a dried blood spot sampling method towards therapeutic monitoring of radotinib in the treatment of chronic myeloid leukaemia. Journal of Clinical Pharmacy and Therapeutics 2020;45(5):1006-1013.
- Population pharmacokinetics of vactosertib, a new new TGF-β receptor type I inhibitor, in patients with advanced solid tumors. Cancer Chemotherapy and Pharmacology 2020;85(1):173-183.
- Pharmacokinetic characteristics of vactosertib, a new activin receptor-like kinase 5 inhibitor, in patients with advanced solid tumors in a first-in-human phase 1 study. Investigational New Drugs 2020;38(3):812-820.
- Comprehensive analyses of safety and efficacy toward individualizing imatinib dosage in patients with chronic myeloid leukemia. International Journal of Hematology 2019;111(3):417-426.
- Association of genetic polymorphisms of CYP2E1, NAT2, GST and SLCO1B1 with the risk of antituberculosis drug-induced liver injury: a systematic review and meta-analysis. BMJ Open 2019;9(8):e027940.
- Determination of a radotinib dosage regimen based on dose-response relationships for the treatment of newly diagnosed patients with chronic myeloid leukemia. Cancer Medicine 2018;7(5):1766-73.
- Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: a systemic review and Bayesian network meta-analysis. Critical Care 2017;21(1):319.
- Optimization of Radotinib Doses for the Treatment of Chronic Myelogenous Leukemia Based on Dose-Response Relationship Analysis. Leukemias & Lymphomas 2016;57(8):1856-64.