취업정보실
프랑스 제약회사 PharmaLex Product Lifecycle Management Consultant 채용
We are looking for remote regulatory support in South Korea. The person needs to be based in KR with a KR cell phone number to access client virtual environment & interact with Ministry of Food and Drug Safety.
Work is fully home based with no travel required. The project begins ASAP and ends 31st July 2023.
It concerns 0. 5 FTE (note that 1 FTE is 1800 billable (operational) hours per year (excluding vacation time, bank holidays, sick leave).
Job description (see attachment for more detail):
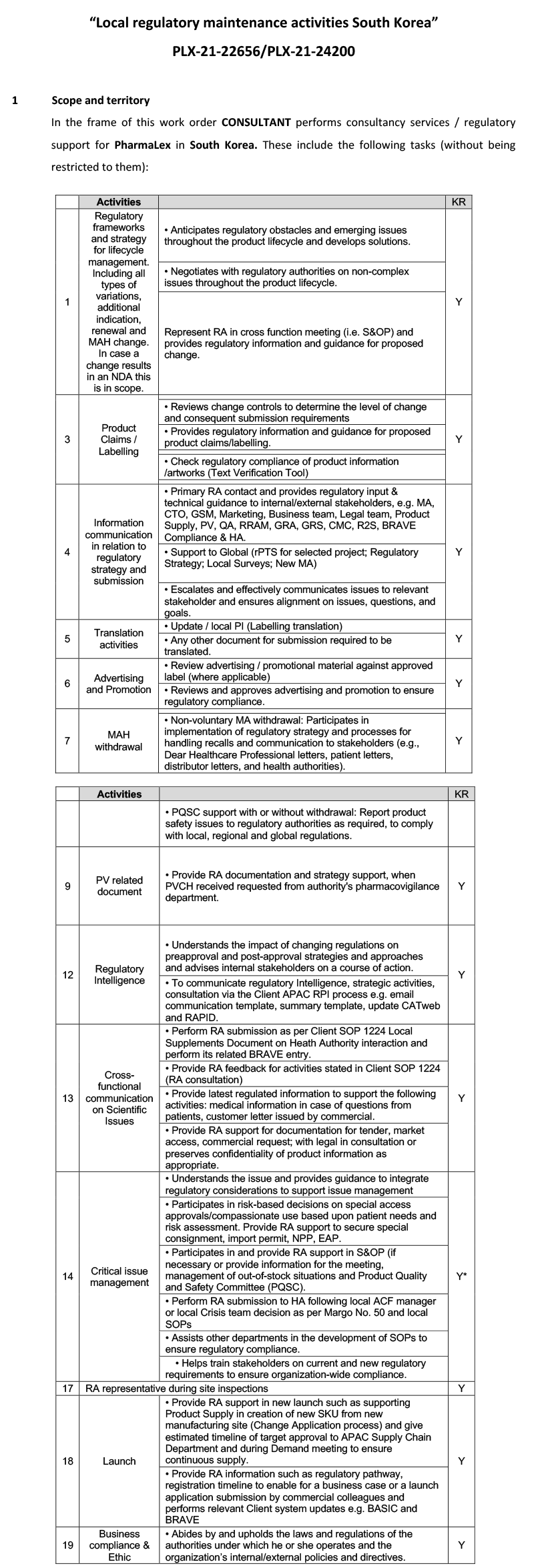
Lifecycle Maintenance activities for 7+ medicinal products (woman health & general medicines):
- Interactions with Korean Health authorities MFDS behalf of our client for dedicated products
- Regulatory frameworks and strategy for lifecycle management. Including all types of variations, additional indication, renewal and MAH change. In case a change in an NDA this is in scope.
- Dossier preparation and submission of hard copy paper and electronic
- Product claims / labelling
- Information communication in relation to regulatory strategy and submission
- Translation activities
- Advertising and promotion
- MAH withdrawal
- Post approval maintenance and reporting
- PV related document
- Invoice check
- Archiving
- Regulatory intelligence
- Cross-functional communication scientific issues
- Critical issue management
- LQSD/LSRP
- Support and response to request form
- and others
Qualification profile:
Pharmacist preferred but BSC or MSc with 2-5 years experience in Pharma industry or health authorities
How to apply:
Please send CV and LinkedIn profile to helene.deconinck@pharmalex.com

